ESCAS confirms efficacy of Cerebrolysin® in aphasia patients
A recently published study on Cerebrolysin® provides promising results for the treatment of aphasia following a stroke.
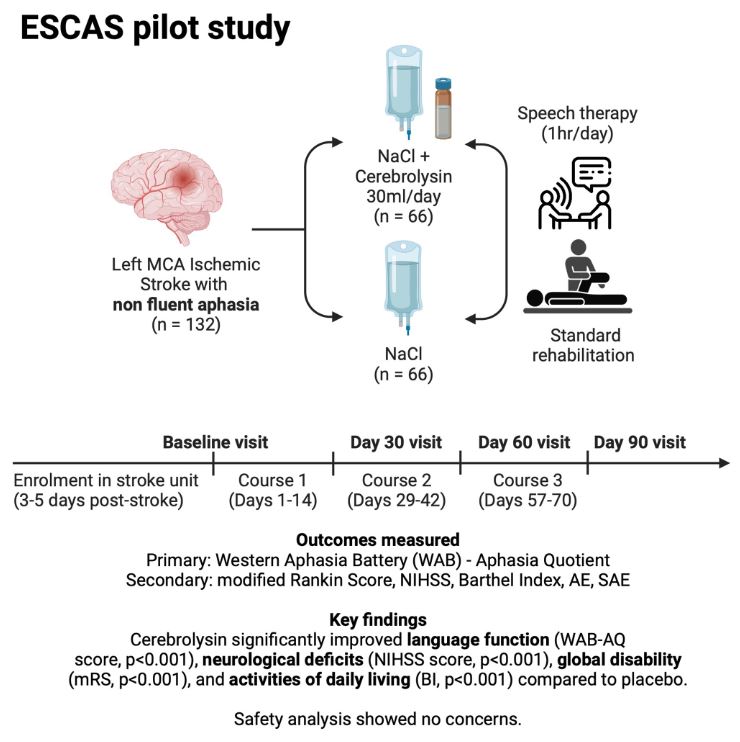
This ESCAS study (Efficacy and Safety of Cerebrolysin in the treatment of Aphasia after Acute Ischemic Stroke) was published in the well-known journal STROKE and demonstrates that a combination therapy of Cerebrolysin® (30ml for 30 days) and speech therapy leads to significantly better therapy outcomes than speech therapy alone.
This prospective, randomized-controlled, double-blinded, multi-center study enrolled 132 patients (66 Cerebrolysin® group, 66 placebo group).
The primary endpoint, the Western Aphasia Battery, shows a large–sized effect size of Cerebrolysin® compared to placebo, statistically significant at day 90. Safety and tolerability observations were comparable between treatment groups.
Please find the whole publication of ESCAS under following link: https://doi.org/10.1161/STROKEAHA.124.049834
A summary of this study you can find in the leaflet below.