New positive trial in acute stroke published
In March 2023 the “CEREbrolysin as an early add-on to reperfusion therapy – risk of HEmorrhagic Transformation after Ischemic Stroke (CEREHETIS)” study was prominently published in BMC Neurology.
It is a prospective, randomized, open-label, active control, multicentre, parallel group study with the objectives to assess the rates of hemorrhagic transformation (primary endpoint), drug safety and functional outcome with 341 enrolled patients. CEREHETIS is a very encouraging study for all acute stroke physicians due to statistically significant outcome of the primary study endpoint (see slide). The NNT supported the clinical relevance of this treatment for physicians and patients alike.
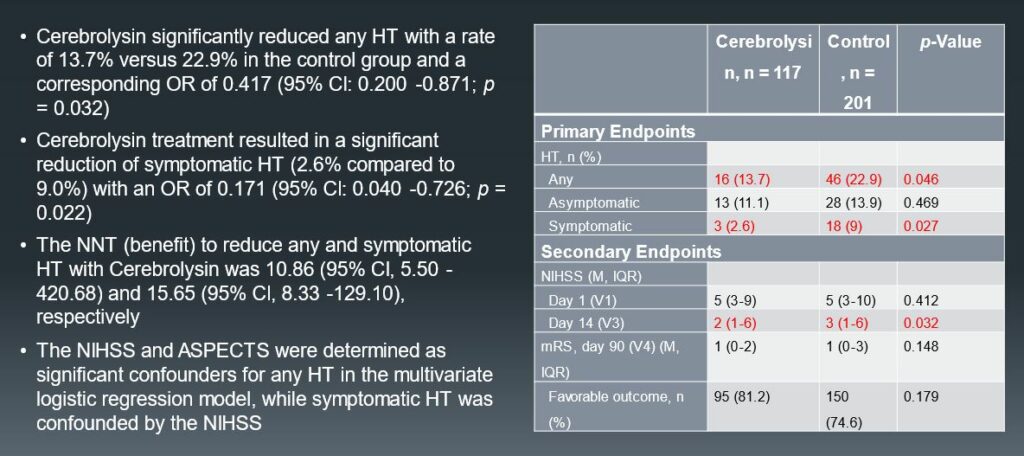
The investigators also analyzed the modified Rankin Score (mRS) on day 90 and observed a trend towards better functional outcome in the Cerebrolysin® group, although both groups showed slightly better 90 day outcome rates than typically observed.
Clinical benefit was also shown in the imaging analyses:
- The laterality index showed a significantly reduced damage in the affected hemisphere after treatment with Cerebrolysin®
- The infarct volume was significantly reduced in the verum group
- The permeability of the blood-brain barrier (BBB) also favored Cerebrolysin-treated patients
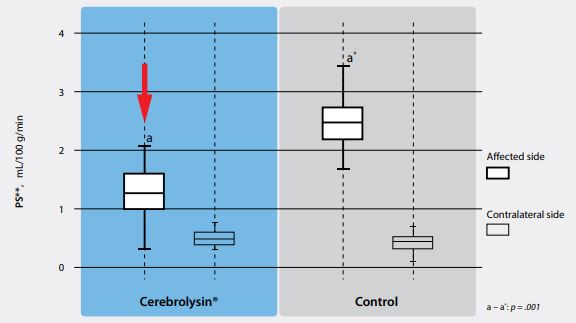
Especially the improvement of the BBB integrity is an important result. This effect has been shown previously in Michael Chopp’s lab at Henry Ford hospital as well as in the published clinical trial by Poljakovic.
Recent data show that Cerebrolysin® increases the safety of recanalization treatment by reducing the frequency of symptomatic and asymptomatic hemorrhagic transformations. In addition, Cerebrolysin® improved the functional outcome, which is of high relevance, given the rapidly developing evidence that any form of hemorrhagic transformation has a negative impact on the functional outcome. Stroke patients undergoing recanalization therapy might therefore benefit from treatment with Cerebrolysin®.