Clinical Efficacy in Stroke
The efficacy and safety of Cerebrolysin® in stroke has been assessed in randomized, double-blind studies with more than 3.000 patients.
Studies showed the following key benefits of Cerebrolysin®
Studies showed the following key benefits of Cerebrolysin®
Cerebrolysin® is safe and well tolerated in both, in ischemic and hemorrhagic stroke.
Early recovery
The early phase of recovery after stroke is very important. Fast improvement of neurological functions is linked with improved stroke prognosis, lower mortality rates and improved motor rehabilitation of the affected extremities. Moreover, it is linked to readiness of stroke patients for neurorehabilitation.
Many studies confirm that Cerebrolysin® treated patients show early recovery effects and patients benefit from an earlier and better starting point for rehabilitation!
Many studies confirm that Cerebrolysin® treated patients show early recovery effects and patients benefit from an earlier and better starting point for rehabilitation!
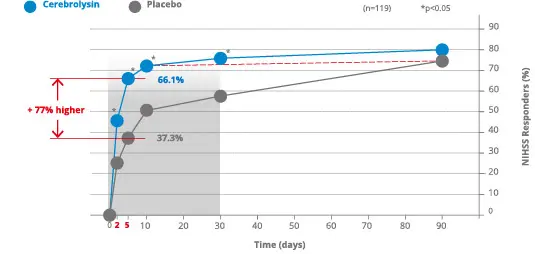
For example, Lang et al. 2013 investigated the combination therapy Cerebrolysin® with alteplase (rtPA). In this study the NIHSS responder rate was investigated. A responder was defined as patient who improved by at least 6 points from baseline, ow whose total score was 0 or 1.
Already on day 2, the NIHSS responder rate was significantly higher with Cerebrolysin® as compared to placebo. The Cerebrolysin® group showed significantly more patients with the defined improvement than in the placebo group. Differences reached its maximum at day 5 with a responder rate of 66.1% in the Cerebrolysin® group vs. 37.3% in the placebo group treated with rtPA alone.
Improvement of motor functions
Stroke often causes serious long-term disabilities. Symptoms are heterogeneous and patients may have widely differing handicaps. One of the ultimate goals in stroke treatment is to improve the patient’s motor functions.
Recently published studies demonstrated that Cerebrolysin® significantly improves the motor function in patients after stroke.
Recently published studies demonstrated that Cerebrolysin® significantly improves the motor function in patients after stroke.
In Muresanu et al. 2016 , the effect size analysis of the ARAT score (primary outcome) demonstrated a large superiority of Cerebrolysin® relative to the placebo on day 90. The ARAT scores increased by median values from 0 at baseline to 51.0 on day 90 in the Cerebrolysin® group and from 2.0 to 27.0 in the placebo group. In the Cerebrolysin® group most patients fully recovered in upper motor functions!
Regained independence
Stroke survivors often suffer from varying degrees of permanent disability and sustain impairments that significantly affect their family and social well-being. Therefore the primary goal after stroke is to regain everyday functions, which include activities of daily living like dressing, feeding, bathing, so that patient can live an independent life.
In the CARS study (Cerebrolysin® and Recovery after Stroke, Muresanu et al. 2016) in which patients participated in an early rehabilitation program incl. Cerebrolysin® treatment a favorable mRS score of 0 (no symptoms) and 1 (no significant disability) was found in 42.3% of patients. In the placebo group only 14.9% reached this level of recovery.
In the CARS study (Cerebrolysin® and Recovery after Stroke, Muresanu et al. 2016) in which patients participated in an early rehabilitation program incl. Cerebrolysin® treatment a favorable mRS score of 0 (no symptoms) and 1 (no significant disability) was found in 42.3% of patients. In the placebo group only 14.9% reached this level of recovery.
Increased quality of life
Post-stroke depression is one of the most serious obstacles for effective recovery and long-term independence. According to epidemiological studies, nearly 30% of stroke patients develop depression, either in the early or in the late stages after stroke. Although depression may affect functional recovery and quality of life after stroke, this condition is often ignored.
In Muresanu et al. 2016 major improvements for depression were observed in the Cerebrolysin® group. As shown by results of the Geriatric Depression Scale, Cerebrolysin® reduces depression and increases the patient’s quality of life.
In Muresanu et al. 2016 major improvements for depression were observed in the Cerebrolysin® group. As shown by results of the Geriatric Depression Scale, Cerebrolysin® reduces depression and increases the patient’s quality of life.
Activation of network plasticity
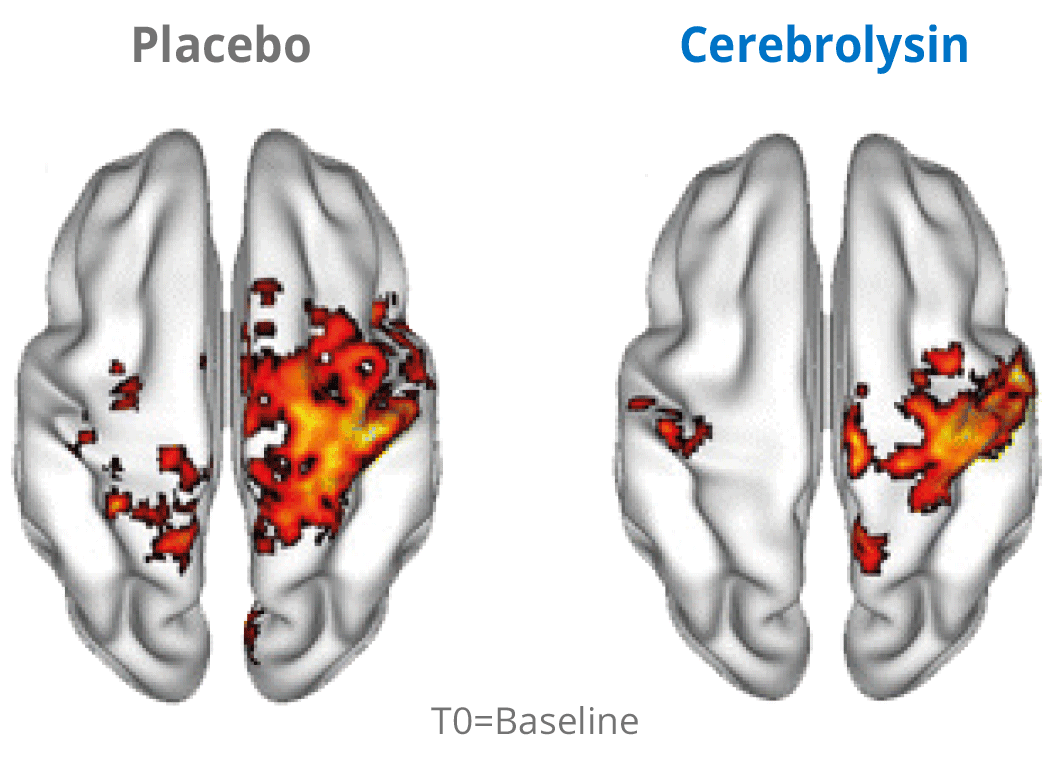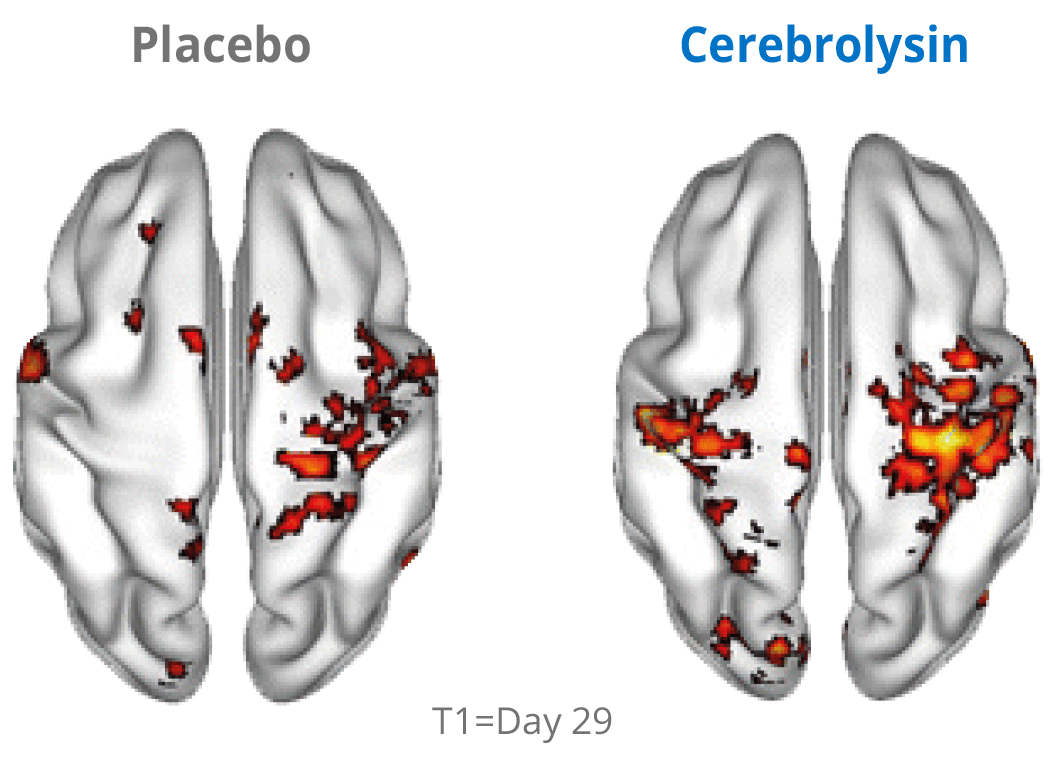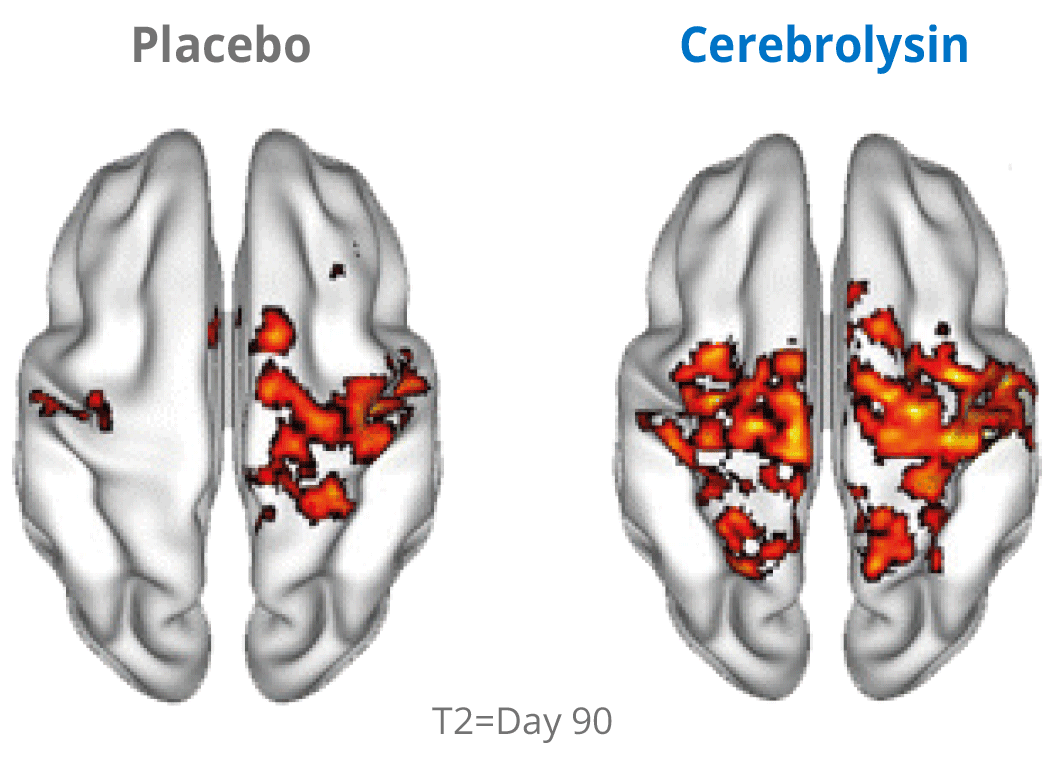
Additional evidence for the effects of Cerebrolysin® on neuroplasticity has been investigated by using functional neuroimaging. The changes in neuroplasticity of the motor network were assessed by resting state functional magnetic resonance imaging (rsfMRI).
Cerebrolysin® treatment induced changes in the sensorimotor network by stimulating symmetric functional connectivity between the bilateral primary sensori-motor cortices. The observed therapeutic effect shows a positive impact of the treatment. The symmetric functional connectivity was more pronounced in patients treated with Cerebrolysin® suggesting better recovery of motor cortical function.
Cerebrolysin® treatment induced changes in the sensorimotor network by stimulating symmetric functional connectivity between the bilateral primary sensori-motor cortices. The observed therapeutic effect shows a positive impact of the treatment. The symmetric functional connectivity was more pronounced in patients treated with Cerebrolysin® suggesting better recovery of motor cortical function.
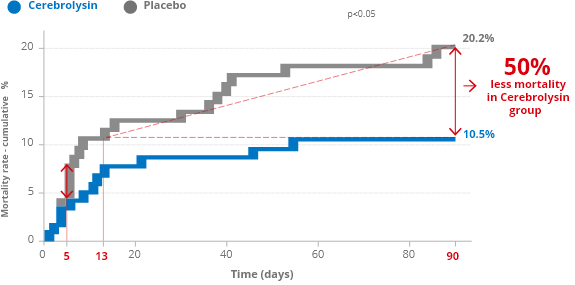
Higher survival rate
Stroke has become second cause of death worldwide. Annually, 5 million people die.
In a multicenter, randomized, double-blind, placebo-controlled trial (CASTA – Cerebrolysin® in patients with Acute ischemic Stroke in Asia –) reported by Heiss et al. 2012 , 1.067 patients with a clinical diagnosis of acute hemispheric ischemic stroke were included. When the mortality of the more severely affected subgroup with NIHSS>12 was analyzed, after 90 days, the cumulative percentage of patients who died was 20.2% in the placebo group but only 10.5% in the Cerebrolysin® group.
In a multicenter, randomized, double-blind, placebo-controlled trial (CASTA – Cerebrolysin® in patients with Acute ischemic Stroke in Asia –) reported by Heiss et al. 2012 , 1.067 patients with a clinical diagnosis of acute hemispheric ischemic stroke were included. When the mortality of the more severely affected subgroup with NIHSS>12 was analyzed, after 90 days, the cumulative percentage of patients who died was 20.2% in the placebo group but only 10.5% in the Cerebrolysin® group.
Double-click this headline to edit the text.
Lang, Wilfried, et al. A prospective, randomized, placebo‐controlled, double‐blind trial about safety and efficacy of combined treatment with alteplase (rt‐PA) and Cerebrolysin® in acute ischaemic hemispheric stroke. International Journal of Stroke, 2013, 8. Jg., nr. 2, S. 95-104.
Muresanu, Dafin F., et al. Cerebrolysin® and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter trial. Stroke, 2016, 47. Jg., nr. 1, S. 151-159.
Chang, Won Hyuk, et al. Cerebrolysin® combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC neurology, 2016, 16. Jg., nr. 1, S. 31.
Heiss, Wolf-Dieter, et al. Cerebrolysin® in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke, 2012, 43. Jg., nr. 3, S. 630-636.
Muresanu, Dafin F., et al. Cerebrolysin® and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter trial. Stroke, 2016, 47. Jg., nr. 1, S. 151-159.
Chang, Won Hyuk, et al. Cerebrolysin® combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC neurology, 2016, 16. Jg., nr. 1, S. 31.
Heiss, Wolf-Dieter, et al. Cerebrolysin® in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke, 2012, 43. Jg., nr. 3, S. 630-636.